Properties of soaps and detergents
Soap – Characteristics And Uses
Soaps are excellent cleansing agents and have good biodegradability. A serious drawback which reduces their general use, is the tendency for the carboxylate ion to react with Ca+ and Mg+ ions in hard water. The result is a water insoluble salt which can be deposited on clothes and other surfaces. These hard water plaques whiten fabric colors and also create rings found in sinks and bath tubs. Another problem with using soaps is their ineffectiveness under acidic conditions. In these cases, soap salts do not dissociate into their component ions, and this renders them ineffective as cleansing agents.
Although primarily used for their cleansing ability, soaps are also effective as mild antiseptics and ingestible antidotes for mineral acid or heavy Metal poisoning. Special metallic soaps, made from soap and heavier metals, are used as additives in polishes, inks, paints, and lubricating oils.
Detergent Physical Characteristics
The concentration at which micelles begin to form is the critical micelle concentration (CMC). The CMC is the maximum monomer concentration and constitutes a measure of the free energy of micelle formation. The lower the CMC, the more stable the micelle and the more slowly Molecules are incorporated into or removed from the micelle. The structure of the hydrophobic region of the detergent can affect the micelle structure. An increase in the length of the hydrophobic hydrocarbon chain of ionic detergents results in an increased micelle size and a lower CMC, as fewer molecules are needed to construct a micelle.
The Average number of monomers in a micelle is the aggregation number. The CMC and aggregation number values are highly dependent on factors such as temperature, pH, ionic strength, and detergent homogeneity and purity. Slight discrepancies in reported values for CMC and aggregation number may be the result of variations in the analytical methods used to determine the values. Aggregation number values are also shifted by concentration, since the number of detergent molecules per micelle may increase if the concentration is above the CMC.
Ease of removal or exchange is an important factor in the selection of a detergent. Some of the more common detergent removal methods include:
- Dialysis
- Gel filtration chromatography
- Hydrophobic adsorption chromatography
- Protein Precipitation
The CMC value associated with the detergent is a useful guide to hydrophobic binding strength. Detergents with higher CMC values have weaker binding and are subsequently easier to remove by dialysis or displacement methods. Detergents with low CMC values require less detergent in order to form micelles and solubilize proteins or lipids.
Another useful parameter when evaluating detergents for downstream removal is the micelle molecular weight, which indicates relative micelle size. Smaller micelles are more easily removed and are usually desirable when protein-detergent complexes are to be separated based on the molecular size of the protein. The micelle molecular weight may be calculated by multiplying the aggregation number by the monomer molecular weight.
The cloud point is the temperature at which the detergent solution near or above its CMC separates into two phases. The micelles aggregate, typically forming a cloudy phase with high detergent concentration, while the balance of the solution becomes detergent-depleted. The resulting two-phase solution can be separated, with the extracted protein being located in the detergent-rich phase. Detergents with low cloud point temperatures, such as TRITON® X-114 (cloud point ~23 °C) are recommended for use with proteins since high cloud point temperatures may denature solubilized proteins. The cloud point can be affected by changes in detergent concentration, temperature, and the addition of salt or polymers such as dextran and polyethylene glycol. Note that the detergent-rich phase is also contingent on the specific detergent(s) and salt concentration; under some conditions the phase may be clear rather than cloudy and be located as either the upper or lower phase of the solution. In non-ionic detergents, this behavior has been applied in the phase separation and purification of membrane proteins.2
Detergent Types and Selection
When selecting a detergent, the first consideration is usually the form of the hydrophilic group:
- Anionic
- Cationic
- Non-ionic
- Zwitterionic (ampholytic)
Anionic and cationic detergents are considered biologically “harsh” detergents because they typically modify protein structure to a greater extent than neutrally charged detergents. The degree of denaturation varies with the individual protein and the particular detergent and concentration. Ionic detergents are more sensitive to pH, ionic strength, and the nature of the counter ion, and can interfere with downstream charge-based analytical methods.
Non-ionic detergents are considered to be “mild” detergents because they are less likely than ionic detergents to denature proteins. By not separating protein-protein Bonds, non-ionic detergents allow the protein to retain its native structure and functionality, although detergents with shorter hydrophobic chain lengths are more likely to cause protein deactivation. Many nonionic detergents can be classified into three structure types:
- Poly(oxyethylene) ethers and related polymers
- Bile salts
- Glycosidic detergents
Poly(oxyethylene) ethers and related detergents have a neutral, polar head and hydrophobic tails that are oxyethylene polymers (e.g. Brij® and TWEEN®) or ethyleneglycoether polymers (e.g. TRITON®). The tert-octylphenol poly(ethyleneglycoether) series of detergents, which includes TRITON X-100 and IGEPAL® CA-630, have an aromatic head that interferes with downstream UV analysis techniques.
Bile salts have a steroid core structure with a polar and apolar orientation, rather than the more obvious nonpolar tail structure of other detergents. Bile salts may be less denaturing than linear chain detergents with the same polar head group.
Glycosidic detergents have a carbohydrate, typically glucose or maltose, as the polar head and an alkyl chain length of 7-14 carbons as the polar tail.
Zwitterionic detergents have characteristics of both ionic and non-ionic detergent types. Zwitterionic detergents are less denaturing than ionic detergents and have a net neutral charge, similar to non-ionic detergents. They are more efficient than non-ionic detergents at disrupting protein-protein bonds and reducing aggregation. These properties have been used for chromatography, mass spectrometry, and electrophoresis methods, and solubilization of organelles and inclusion bodies.
Non-detergent sulfobetaines (NDSB), although not detergents, possess hydrophilic groups similar to those of zwitterionic detergents but with shorter hydrophobic chains. Sulfobetaines do not form micelles. They have been reported to improve the yield of membrane proteins when used with detergents and prevent aggregation of denatured proteins.
Uses for Detergent
Powder and liquid detergent can be used for other purposes besides cleaning clothes or dishes. This can save you Money from having to buy multiple cleaning products.
All-Purpose Cleaner
Either form of detergent can be used to clean tiles, floors, counters, tubs and toilets. Mix 3/4 of a cup of bleach, 1 cup of detergent and 1 gallon of hot water together and pour it into spray bottles for a supply of all-purpose cleaner.
Moss Killer
Sprinkle powdered detergent on moss that is growing in the cracks of your steps, sidewalk or driveway. Give it a few days to turn brown, then brush it from the cracks with a broom.
Oil Spills
Powdered detergent can absorb oil that’s spilled on a garage floor or on the street.
Carpet Cleaning
Both types of cleaner can be added to carpet steam cleaners to make the carpet smell fresher and boost the appliance’s cleaning power.
Drains
Instead of buying Drano to clean out a drain, put 1/4 cup of liquid detergent into the drain, then pour in a boiling pot of water after a minute to flush out to blockage.
Bubbles
If kids like to make bubbles with wants or play with bubble-making guns, one can make the bubble solution oneself by mixing liquid detergent with water.
,
Soaps and detergents are two types of cleaning agents that are used to remove dirt, grease, and other impurities from surfaces. They work by lowering the Surface Tension of water, which allows them to more easily penetrate and remove these substances. Soaps are made from natural fats or oils, while detergents are made from synthetic chemicals.
Soaps and detergents have a number of properties that make them effective cleaning agents. These properties include solubility, surface tension, emulsification, detergency, cleansing action, foaming, hard water Tolerance, skin irritation, biodegradability, ecotoxicity, economic impact, and future trends.
Solubility: Soaps and detergents are soluble in water. This means that they can be mixed with water to form a solution. The solubility of soaps and detergents is affected by the temperature of the water and the type of soap or detergent being used.
Surface tension: Surface tension is the force that acts at the surface of a liquid and causes it to behave like an elastic skin. Soaps and detergents lower the surface tension of water, which allows them to more easily penetrate and remove dirt, grease, and other impurities from surfaces.
Emulsification: Emulsification is the process of combining two liquids that do not normally mix, such as oil and water. Soaps and detergents are emulsifiers, which means that they can help to create an emulsion of oil and water. This is why soaps and detergents are often used to clean greasy surfaces.
Detergency: Detergency is the ability of a substance to remove dirt, grease, and other impurities from surfaces. Soaps and detergents are detergents, which means that they are effective at removing these substances.
Cleansing action: Cleansing action is the process of removing dirt, grease, and other impurities from surfaces. Soaps and detergents work by lowering the surface tension of water, which allows them to more easily penetrate and remove these substances. Soaps and detergents also contain surfactants, which are molecules that have both water-loving and oil-loving ends. The water-loving ends of the surfactants attach to the water molecules, while the oil-loving ends of the surfactants attach to the dirt, grease, and other impurities. This helps to loosen and remove these substances from the surface.
Foaming: Foaming is the formation of bubbles when a liquid is agitated. Soaps and detergents are foaming agents, which means that they can help to create bubbles. Foaming can be helpful in cleaning because it helps to trap dirt, grease, and other impurities.
Hard water tolerance: Hard water is water that contains Minerals such as calcium and magnesium. These minerals can interfere with the ability of soaps and detergents to work effectively. Soaps and detergents that are designed for use in hard water contain ingredients that help to prevent these minerals from interfering with their performance.
Skin irritation: Soaps and detergents can irritate the skin, especially if they are used frequently or if they are not rinsed off completely. This is because soaps and detergents can remove the natural oils from the skin, which can lead to dryness, itching, and redness.
Biodegradability: Biodegradability is the ability of a substance to be broken down by Microorganisms. Soaps and detergents are biodegradable, which means that they will eventually break down into harmless substances.
Ecotoxicity: Ecotoxicity is the harmful effects of a substance on the Environment. Soaps and detergents can be harmful to the environment if they are not disposed of properly. They can pollute waterways and harm aquatic life.
Economic impact: The economic impact of soaps and detergents is significant. The global market for soaps and detergents is worth billions of dollars. Soaps and detergents are used in a variety of industries, including the personal care Industry, the household cleaning industry, and the industrial cleaning industry.
Future trends: The future trends for soaps and detergents are likely to focus on the development of more environmentally friendly products. There is also likely to be a trend towards the use of natural soaps and detergents.
What are soaps and detergents?
Soaps and detergents are both surfactants, which means they lower the surface tension of water. This allows them to dissolve in water and interact with other substances, such as dirt and oil. Soaps are made from animal or vegetable fats or oils that have been reacted with an alkali, such as sodium hydroxide or potassium hydroxide. Detergents are synthetic chemicals that are designed to mimic the properties of soaps.
How do soaps and detergents work?
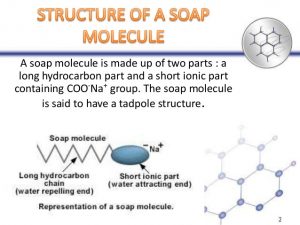
Soaps and detergents work by reducing the surface tension of water. This allows them to wet surfaces more easily and to penetrate into pores and crevices. They also surround dirt and oil particles, forming micelles. Micelles are tiny spheres with the dirt and oil particles trapped inside. The water-soluble ends of the soap or detergent molecules are on the outside of the micelle, while the oil-soluble ends are on the inside. This allows the micelles to be easily rinsed away with water.
What are the benefits of using soaps and detergents?
Soaps and detergents can help to remove dirt, oil, and other substances from surfaces. They can also help to kill bacteria and other microorganisms. Soaps and detergents are also relatively inexpensive and easy to use.
What are the risks of using soaps and detergents?
Soaps and detergents can be irritating to the skin, especially if they are used in high concentrations. They can also be harmful to the environment, especially if they are not disposed of properly. Soaps and detergents can also pollute waterways and harm aquatic life.
How can I use soaps and detergents safely?
To use soaps and detergents safely, it is important to follow the directions on the label. It is also important to avoid getting soaps and detergents in your eyes. If you do get soap or detergent in your eyes, rinse them immediately with water. If you experience any irritation, redness, or swelling, see a doctor.
What are some alternatives to soaps and detergents?
There are a number of alternatives to soaps and detergents, such as baking soda, vinegar, and lemon juice. These alternatives can be used to clean surfaces and remove dirt and oil. However, they may not be as effective as soaps and detergents.
What are some common mistakes people make when using soaps and detergents?
Some common mistakes people make when using soaps and detergents include using too much soap or detergent, not rinsing soap or detergent off completely, and using soaps and detergents that are not appropriate for the surface they are cleaning.
What are some tips for choosing the right soap or detergent?
When choosing a soap or detergent, it is important to consider the type of surface you are cleaning, the amount of dirt and oil you need to remove, and your skin type. It is also important to choose a soap or detergent that is biodegradable and environmentally friendly.
Which of the following is not a property of soaps and detergents?
(A) They are both surfactants.
(B) They both have a long hydrocarbon chain and a short polar group.
(C) Soaps are made from animal fats or vegetable oils, while detergents are made from petroleum products.
(D) Soaps are more effective in hard water than detergents.Which of the following is the main difference between soaps and detergents?
(A) Soaps are made from animal fats or vegetable oils, while detergents are made from petroleum products.
(B) Soaps are more effective in hard water than detergents.
(C) Soaps are anionic surfactants, while detergents are either anionic, cationic, or nonionic surfactants.
(D) Soaps are biodegradable, while detergents are not.Which of the following is an example of a soap?
(A) Sodium stearate
(B) Sodium lauryl sulfate
(C) Sodium dodecylbenzene sulfonate
(D) All of the aboveWhich of the following is an example of a detergent?
(A) Sodium stearate
(B) Sodium lauryl sulfate
(C) Sodium dodecylbenzene sulfonate
(D) None of the aboveSoaps and detergents work by:
(A) lowering the surface tension of water.
(B) forming micelles around dirt particles.
(C) both (A) and (B).
(D) neither (A) nor (B).Hard water contains:
(A) calcium and magnesium ions.
(B) sodium and potassium ions.
(C) chloride and sulfate ions.
(D) all of the above.Soaps are less effective in hard water because:
(A) the calcium and magnesium ions form insoluble salts with the soap molecules.
(B) the calcium and magnesium ions compete with the soap molecules for binding to dirt particles.
(C) both (A) and (B).
(D) neither (A) nor (B).Detergents are more effective in hard water than soaps because:
(A) they do not form insoluble salts with the calcium and magnesium ions.
(B) they are not affected by the calcium and magnesium ions.
(C) both (A) and (B).
(D) neither (A) nor (B).Soaps are biodegradable, while detergents are not. This means that:
(A) soaps will break down into harmless substances in the environment, while detergents will not.
(B) soaps will not harm the environment, while detergents will.
(C) both (A) and (B).
(D) neither (A) nor (B).Which of the following is a disadvantage of using soaps?
(A) They are not biodegradable.
(B) They can cause skin irritation in some people.
(C) They can form scum in hard water.
(D) All of the above.Which of the following is a disadvantage of using detergents?
(A) They are not biodegradable.
(B) They can cause skin irritation in some people.
(C) They can form scum in hard water.
(D) All of the above.Which of the following is a sustainable alternative to soaps and detergents?
(A) Borax
(B) Washing soda
(C) Castile soap
(D) All of the above.