Preparation of Oxygen
Oxygen is prepared in lab generally in two ways either by the application of heat or no application of heat.
Using heat:
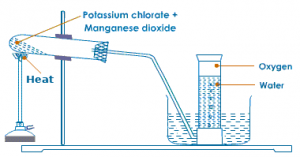
Oxygen in lab is prepared by heating the mixture of powdered potassium chlorate and manganese dioxide in the ratio 4:1 in a hard glass test tube. The oxygen gas is observed in a gas jar through the downward displacement of water. The reaction involved is given below:
2KClO3−→−−−−−MnO2200−300∘C2KCl+3O2↑
Without heat:
The dry sodium peroxide is taken in a conical flask and the apparatus is fitted as shown in figure. The two necks of woulfe’s bottle is tighten up with cork so that no air enters from outside. It is connected with the delivery tube to a water trough containing water. Water is poured slowly from the thistle funnel. Here sodium peroxide reacts with water at ordinary temperature to give hydrogen gas. The hydrogen gas thus formed is collected in a gas jar through delivery tube by the downward displacement of water. The reaction takes place as below:
2Na2O2+2H2O⟶4NaOH+O2↑
Physical properties of oxygen:
– Oxygen is colorless, odourless and tasteless gas.
– It is pale blue color in liquid and solid state.
– It is slightly soluble in water.
– It is heavier than air.
Chemical properties of oxygen:
Combustibility: It does not burn itself but it supports for combustion. Oxygen requires high initial heating due to its high bond dissociation energy of 493.4KJmol– between the O=O atoms.
Action with hydrogen: Oxygen when heated with hydrogen forms water.
2H2+O2−→Δ2H2O2H2+O2→Δ2H2O
Action with Nitrogen: Oxygen reacts with nitrogen at high temperature to give nitrogen dioxide.
N2+O2−→−−−3000∘C2NON2+O2→3000∘C2NO
NO+O2⟶2NO2NO+O2⟶2NO2
Action with carbon: Carbon when reacted with limited oxygen gives carbon monoxide and with excess oxygen, carbon gives carbon dioxide.
2C+O2limited⟶COCarbon Monoxide2C+O2limited⟶COCarbon Monoxide
2C+O2Excess⟶CO2Carbon Monoxide2C+O2Excess⟶CO2Carbon Monoxide
Action with ammonia: Oxygen reacts with ammonia to give nitric oxide and water. It is a reaction involved in manufacture of nitric acid in Ostwald’s process.
4NH3+5O2−→−−−Pt/MO800∘C4NO+6H2O↑4NH3+5O2→Pt/MO800∘C4NO+6H2O↑
Action with glucose: Glucose reacts with oxygen to give carbon dioxide, water and energy. This reaction takes place inside the human body.
Glucose + Oxygen→Carbon Dioxide + Water + EnergyGlucose + Oxygen→Carbon Dioxide + Water + Energy
i.e., C6H12O6+6O2⟶6CO2+6H2O+EnergyC6H12O6+6O2⟶6CO2+6H2O+Energy
Action with metals:
Oxygen combines with many metals to form their respective oxides.
4Na+O2−→−−−−−−−−−Room temperature2NaO24Na+O2→Room temperature2NaO2
2Na+O2−→−−300∘CNa2O22Na+O2→300∘CNa2O2
4K+O2⟶2K2O4K+O2⟶2K2O
2Mg+O2−→Δ2MgO2Mg+O2→Δ2MgO
2Zn+O2−→Δ2ZnO2Zn+O2→Δ2ZnO
4Al+3O2−→ΔAl2O34Al+3O2→ΔAl2O3
Action with iron: Oxygen when reacted with iron gives ferrous oxide. When excess of oxygen is passed, it gives ferrosoferic oxide and further addition of oxygen gives ferric oxide. To be discused
Fe+O2−→ΔFeOFerrousoxideFe+O2→ΔFeOFerrousoxide
6FeO+O2−→Δ2Fe3O4Ferrosofericoxide6FeO+O2→Δ2Fe3O4Ferrosofericoxide
4Fe+3O2−→Δ2Fe3O3Ferricoxide4Fe+3O2→Δ2Fe3O3Ferricoxide
When oxygen is reacted with oxygen in presence of water, rust is formed.
4Fe+3O2+2H2O⟶2Fe2O3⋅XH2ORust4Fe+3O2+2H2O⟶2Fe2O3⋅XH2ORust
Uses of oxygen:
– It is used for artificial Respiration in hospitals, mountaineers in high altitude, miners and sea divers in the form of oxygen mask.
– It is used as aero fuel in rocket engines and planes.
– It is used for the generation of energy inside our body.
– It is used as strong oxidizing agent in laboratory.
– It is used by the Plants for the process of Photosynthesis.
– It is used in preparing different explosives.
– It is used as a germicides and insecticides.
– It is the main element for the formation of ozone.
,
Distillation of Liquid Air
Distillation is a process of separating mixtures based on their boiling points. Liquid air is a mixture of nitrogen and oxygen, and the boiling points of these two gases are very different. Nitrogen has a boiling point of -195.8 degrees Celsius, while oxygen has a boiling point of -183 degrees Celsius. This means that if liquid air is heated, the nitrogen will vaporize first, followed by the oxygen.
Distillation of liquid air is a common industrial process. It is used to produce pure nitrogen and oxygen, which are used in a variety of applications, including welding, Food Processing, and medical research.
Electrolysis of Water
Electrolysis is a process of using electricity to separate the components of a compound. In the case of water, electrolysis separates water into hydrogen and oxygen gas.
Electrolysis of water is a simple process. Water is placed in an electrolytic cell, which contains two electrodes. An electric current is passed through the water, and the water Molecules are split into hydrogen and oxygen gas.
The hydrogen and oxygen gas are collected at the electrodes. The hydrogen gas is collected at the cathode, and the oxygen gas is collected at the anode.
Electrolysis of water is a clean and efficient way to produce hydrogen and oxygen gas. It is used in a variety of applications, including Fuel Cells, water treatment, and the production of ammonia.
Decomposition of Potassium Chlorate
Potassium chlorate is a white solid that is used in a variety of applications, including fireworks, matches, and explosives. Potassium chlorate can be decomposed into potassium chloride and oxygen gas by heating it to a high temperature.
The decomposition of potassium chlorate is a Chemical Reaction. The chemical equation for the reaction is:
2KClO3(s) 2KCl(s) + 3O2(g)
The decomposition of potassium chlorate is an exothermic reaction, which means that it releases heat. The heat released by the reaction can be used to ignite other materials, such as gunpowder.
Decomposition of Sodium Chlorate
Sodium chlorate is a white solid that is used in a variety of applications, including bleach, explosives, and fireworks. Sodium chlorate can be decomposed into sodium chloride and oxygen gas by heating it to a high temperature.
The decomposition of sodium chlorate is a chemical reaction. The chemical equation for the reaction is:
2NaClO3(s) 2NaCl(s) + 3O2(g)
The decomposition of sodium chlorate is an exothermic reaction, which means that it releases heat. The heat released by the reaction can be used to ignite other materials, such as gunpowder.
Decomposition of Hydrogen Peroxide
Hydrogen peroxide is a colorless liquid that is used in a variety of applications, including cleaning, bleaching, and hair care. Hydrogen peroxide can be decomposed into water and oxygen gas by heating it to a high temperature or by adding a Catalyst.
The decomposition of hydrogen peroxide is a chemical reaction. The chemical equation for the reaction is:
2H2O2(l) 2H2O(l) + O2(g)
The decomposition of hydrogen peroxide is an exothermic reaction, which means that it releases heat. The heat released by the reaction can be used to ignite other materials, such as gunpowder.
Thermal Decomposition of Metal Oxides
Metal oxides are compounds that are formed when a metal reacts with oxygen. Metal oxides can be decomposed into the metal and oxygen gas by heating them to a high temperature.
The decomposition of metal oxides is a chemical reaction. The chemical equation for the reaction is:
MO(s) M(s) + O2(g)
The decomposition of metal oxides is an exothermic reaction, which means that it releases heat. The heat released by the reaction can be used to melt the metal or to ignite other materials.
Photochemical Decomposition of Water
Photochemical decomposition of water is a process that uses Light to break down water into hydrogen and oxygen gas. The process is carried out in a chamber that is filled with water and a catalyst. The catalyst helps to speed up the reaction.
When light is shone on the water, it excites the electrons in the water molecules. The excited electrons then react with the hydrogen ions in the water to form hydrogen gas. The oxygen ions in the water then react with each other to form oxygen gas.
The photochemical decomposition of water is a clean and efficient way to produce hydrogen and oxygen gas. It is used in a variety of applications, including fuel cells, water treatment, and the production of ammonia.
What is oxygen?
Oxygen is a chemical element with the symbol O and atomic number 8. It is the third most abundant element in the universe by mass, after hydrogen and helium. It is the second most abundant element in the Earth’s Atmosphere, at 20.95%. It is a member of the chalcogen group on the periodic table and is a highly reactive non-metal.
What are the uses of oxygen?
Oxygen is used in a variety of applications, including:
- Respiration: Oxygen is essential for respiration, the process by which cells convert food into energy.
- Combustion: Oxygen is required for combustion, the process by which fuel is burned.
- Welding: Oxygen is used in welding to create a strong bond between two pieces of metal.
- Cutting: Oxygen is used in cutting to create a clean, precise cut.
- Medical: Oxygen is used in hospitals to treat patients with respiratory problems.
How is oxygen produced?
Oxygen is produced by a variety of methods, including:
- Fractional distillation: Oxygen is produced by fractional distillation of liquid air.
- Electrolysis: Oxygen is produced by electrolysis of water.
- Photosynthesis: Oxygen is produced by photosynthesis, the process by which plants use sunlight to convert carbon dioxide and water into glucose and oxygen.
What are the safety precautions for oxygen?
Oxygen is a highly flammable gas and should be handled with care. It is important to avoid sources of ignition, such as open flames and sparks. Oxygen should also be stored in a cool, dry place.
What are the environmental impacts of oxygen?
Oxygen is not considered to be a major environmental pollutant. However, the release of oxygen into the atmosphere can contribute to Climate change.
What are the future trends for oxygen?
The demand for oxygen is expected to increase in the future due to the Growth of the global Population and the increasing use of oxygen in medical and industrial applications.
Which of the following is not a method of preparing oxygen?
(A) Electrolysis of water
(B) Thermal decomposition of potassium chlorate
(C) Decomposition of ozone
(D) Distillation of liquid airThe correct balanced equation for the decomposition of potassium chlorate is:
(A) 2KClO3(s) 2KCl(s) + 3O2(g)
(B) 2KClO3(s) 2KCl(s) + O2(g)
(C) 2KClO3(s) 2KCl(aq) + 3O2(g)
(D) 2KClO3(s) 2KCl(aq) + O3(g)The correct balanced equation for the electrolysis of water is:
(A) 2H2O(l) 2H2(g) + O2(g)
(B) 2H2O(l) H2(g) + 2OH-(aq)
(C) 2H2O(l) 2H+ (aq) + 2OH-(aq)
(D) 2H2O(l) 2H+ (aq) + O2(g) + 2e-The correct balanced equation for the decomposition of ozone is:
(A) 2O3(g) 3O2(g)
(B) 3O3(g) 2O2(g) + O(g)
(C) 2O3(g) O2(g) + O2(g)
(D) 2O3(g) 3O2(g) + O3(g)The correct balanced equation for the distillation of liquid air is:
(A) N2(l) + O2(l) N2(g) + O2(g)
(B) 2N2(l) + O2(l) 2N2(g) + O2(g)
(C) N2(l) + O2(l) 2N2(g) + O3(g)
(D) 2N2(l) + O2(l) 2N2(g) + 3O2(g)The density of oxygen at STP is:
(A) 1.429 g/L
(B) 1.293 g/L
(C) 1.184 g/L
(D) 1.000 g/LThe melting point of oxygen is:
(A) -218.4°C
(B) -219.6°C
(C) -220.0°C
(D) -221.0°CThe boiling point of oxygen is:
(A) -183°C
(B) -184°C
(C) -185°C
(D) -186°CThe critical temperature of oxygen is:
(A) -118.4°C
(B) -119.6°C
(C) -120.0°C
(D) -121.0°CThe critical pressure of oxygen is:
(A) 50.1 atm
(B) 51.1 atm
(C) 52.1 atm
(D) 53.1 atm