laboratory method of preparing alcohol
Hydrolysis of Alkyl Halides
This is a nucleophilic substitution reaction.
R-X + KOHaq → R-OH
The method is not satisfactory as olefins are also formed as by-products. However better yields is obtained by using moist Ag2O or aqueous K2CO3. Tertiary butyl halides mainly gives alkene due to dehydrohalogenation.
Hydration of Alkenes
This is electrophilic addition of H2O to alkenes.
Mechanism of Hydration of alkenes:
Protonation of alkene to form carbocation by electrophilic
Nucleophilic attack of water on carbocation.
Deprotonation to form an alcohol.
Except ethyl alcohol no other primary alcohol can be obtained by this method, however hydroboration of terminal alkenes give primary alcohols.
Oxymercuration and Demercuration of Alkanes
Alkenes react with mercuric acetate in presence of H2O and tetra hydrofuran to give alkyl mercury compounds.
Examples:
Hydroboration Oxidation
From Grignard Reagents
All the three types of monohydric alcohols (primary, secondary and tertiary alcohols) are obtained by the use of Grignard reagents and carbonyl compounds. The addition of RMgX on carbonyl compounds followed with hydrolysis yields alcohols.
The Grignard reagent : an organometallic compound
When a solution of an alkyl halide in dry ethyl ether, (C2H5)O, is allowed to stand over turnings of metallic magnesium a vigorous reaction takes place: the solution turns cloudy, begins to boil, and the magnesium Metal gradually disappears. The resulting solution is known as a Grignard reagent, after Victor Grignard (of the University of Lyons) who received the Nobel prize in 1912 for its discovery. It is one of the most useful and versatile reagents known to the organic chemist.
CH3I + Mg CH3MgI
H3CH2Br + Mg CH3CH2MgBr
Ethyl bromide Ethylmagnesium bromide
The Grignard reagent has the general formula R MgX, and the general name alkylmagnesium halide. The carbon-magnesium bond is covalent but highly polar, with carbon pulling electrons from electropositive magnesium; the magnesium halogen bond is essentially ionic. R–Mg+X
Since magnesium becomes bonded to the same carbon that previously held halogen, the alkyl group remains intact during the preparation of the reagent. Thus n-propyl chloride yields n-propylmagnesium chloride, and isopropyl chloride yields isopropylmagnesium chloride.
CH3CH2CH2Cl + Mg CH3CH2CH2MgCl
n-Propyl chloride n-Propylmagnesium chloride
CH3CHClCH3 + Mg CH3CHMgClCH3
Isopropyl chloride Isopropylmagnesium chloride
The Grignard reagent is the best-known member of a broad class of substances, called organometallic compounds, in which carbon is bonded to a metal: lithium potassium, sodium, zinc, mercury, lead, thallium-almost any metal known. Each kind of organometallic compound has, of course, its own set of properties, and its particular uses depend on these. But whatever the metal, it is less elctronegative than carbon, and the carbon-metal bond-like one in the Grignard reagent – is highly polar. Although the organic group is not a full-fledged carbanion–an anion in which carbon carries negative charge–it nevertheless has considerable carbanion character. As we shall see, organometallic compounds owe their enormous usefulness chiefly to one common quality: they can serve as a source from which carbon is readily transferred with its electrons.
The Grignard reagent is highly reactive. It reacts with numerous inorganic compounds including water, carbon dioxide, and Oxygen, and with most kinds of organic compounds; in many of these cases the reaction provides the best way to make a particular class of organic compounds.
The reaction with water to form an alkane is typical of the behaviour of the Grignard reagent–and many of the more reactive organometallic compounds–toward acids. In view of the marked carbanion character of the alkyl group, we may consider the Grignard reagent to be the magnesium salt, R MgX, of the extremely weak acid,
R–H. The reaction
R MgX + HOH → R–H + Mg(OH)X
Stronger Weaker
acid acid
is simply the displacement of the weaker acid, R–H, from its salt by the stronger acid, HOH.
R MgX + NH3 → R–H + Mg(NH2)X
Stronger Weaker
acid acid
An alkane is such a weak acid that it is displaced from the Grignard reagent by compounds that we might ordinarily consider to be very weak acids themselves, or possibly not acids at all. Any compound containing hydrogen attached to oxygen or nitrogen is tremendously more acidic than an alkane, and therefore can decompose the Grignard reagent: for example, ammonia or methyl alcohol.
RMgX + CH3OH → R–H + Mg(OCH3)X
Stronger Weaker
acid acid
Grignard Synthesis of Alcohols
The Grignard reagent, we recall, has the formula RMgX, and is prepared by the reaction of metallic magnesium with the appropriate organic halide. This halide can be alkyl (1o, 2o, 3o), allylic, aryl alkyl (e.g., benzyl), or aryl (phenyl) or substituted phenyl. The halogen may be –Cl, –Br or –I, (Arylmagnesium chlorides must be made in the cyclic ether tetrahydrofuran instead of ethyl ether.)
Aldehydes and ketones resemble each other closely in most of their reactions. Like the carbon-carbon double bond, the carbonyl group is unsaturated, and like the carbon-carbon bond, it undergoes addition. One of its typical reactions is addition of the Grignard reagent.
Since the electrons of the carbonyl double bond hold together atoms of quite different electronegativity, we would not expect the electrons to be equally shared; in particular, the mobile p cloud should be pulled strongly towards the more electronegative atom, oxygen. Whatever the mechanism involved, addition of an unsymmetrical reagent is oriented so that the nucleophilic (basic) portion attaches itself to carbon, and the electrophilic (acidic) portion attaches itself to oxygen.
The carbon-magnesium bond of the Grignard reagent is a highly polar bond, carbon being negative relative to electropositive magnesium. It is not surprising, then, that in the addition to carbonyl compounds, the organic group becomes attached to carbon and magnesium to oxygen. The product is the magnesium
salt of the weakly acidic alcohol and is easily converted into the alcohol itself by the addition of the stronger acid, water. Since the Mg(OH)X thus formed is a gelatinous material difficult to handle, dilute mineral acid (HCl, H2SO4) is commonly used instead of water, so that water-soluble magnesium salts are formed.
Products of the Grignard Synthesis
The class of alcohol that is obtained from a Grignard synthesis depends upon the type of carbonyl compoud used: formaldehyde, HCHO, yields primary alcohols; other aldehydes, RCHO, yield secondary alcohols; and ketones, R2CO, yield tertiary alcohols.
This relationship arises directly from our definitions of aldehydes and ketones, and our definitions of primary, secondary, and tertiary alcohols. The number of hydrogens attached to the carbonyl carbon defines the carbonyl compound as formaldehyde, higher aldehyde or ketone. The carbonyl carbon is the one that finally bears the –OH group in the product; here the number of hydrogen defines the alcohol as primary, secondary, or tertiary.
For example:
A related synthesis utilized ethylene oxide to make primary alcohols containing two more carbons than the Grignard reagent.
Here, too, the organic group becomes attached to carbon and magnesium to oxygen, this time with the breaking of a carbon-oxygen s bond in the highly strained three-membered ring. For example:
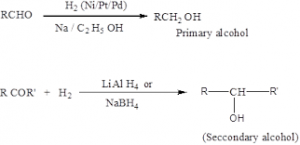
Reduction of Carbonyl Compounds
Aldehydes can be reduced to primary alcohols, and ketones to secondary alcohols, either by catalytic hydrogenation or by use of chemical reducing agents like lithium aluminum hydride, LiAlH4. Such reduction is useful for the preparation of certain alcohols that are less available than the corresponding carbonyl compounds, in particular carbonyl compounds that can be obtained by the aldol condensation. For example
Reduction of ketones gives secondary alcohol.
Note : tertiary alcohols can be obtained by this method.
Sodium borohydride, NaBH4, does not reduce carbon-carbon double Bonds, not even those conjugated with carbonyl groups, and in thus useful for the reduction of such unsaturated carbonyl compounds to unsaturated alcohols.Let us look a little more closely at reduction by metal hydrides. Alcohols are formed from carbonyl compounds, smoothly and in high yield, by the action of such compounds as lithium aluminum hydride, LiAlH4. Here again, we see
Nucleophilic addition : this time the nucleophile is hydrogen transferred with a pair of electrons-as a hydride ion, H:– –from the metal to carbonyl carbon:
Reduction of acids to alcohols: Lithium aluminum hydride, LiAlH4, is one of the few reagents that can reduce an acid to an alcohol; the inital product is an alkoxide from which the alcohol is liberated by hydrolysis:
4RCOOH + 3LiAlH4 → 4RCH2OH 1oalcohol
Because of the excellent yields it gives, LiAlH4 is widely used in the laboratory for the reduction of not only acids but many other classes of compounds. As an alternative to direct reduction, acids are often converted into alcohols by a two-step process: esterification, and reduction of the ester.
Reduction of esters: Like many organic compounds, esters can be reduced in two ways: (A) by catalytic hydrogenation using molecular hydrogen, or (B) chemical reduction. In either case, the ester is cleaved to yield (in addition to the alcohol or phenol from which it was derived) a primary alcohol corresponding to the acid portion of the ester.
RCOOR’ RCH2OH + R’OH
Ester 1o alcohol
Hydrogenolysis (cleavage by hydrogen) of an ester requires more severe conditions than simple hydrogenation of (addition of hydrogen to) a carbon-carbon double bond. High pressures and elevated temperatures are required: the Catalyst used most often is a mixture of oxides known as copper chromite, of approximately the composition CuO.CuCr2O4. For example:
CH3(CH2)10COOCH3 CH3(CH2)10CH2OH + CH3OH
(Methyl dodecanoate) (1-Dodecanol)
Chemical reduction is carried out by use of sodium metal and alcohol, or more usually by use of lithium aluminium hydride
By the reduction of acids and their Derivatives :
RCOOH RCH2OH
(RCO2)O RCH2OH
RCOCI RCH2OH
RCOOR’ RCH2OH + R’OH
Note : If C2H5OH + Na is used as reducing agent, the reduction is known as Bouveault-Blane reaction.
By the action of nitrous acid on primary amines :
R-NH2 + HNO2 → R-OH + N2 + H2O
However under similar conditions CH3NH2 gives CH3-O-N=O or CH3OCH3
CH3NH2 + 2HNO2 → CH3-O-N=O + 2H2O + N2
or 2CH3NH2 + 2HNO2 → CH3OCH3 + 2N2 + 3H2O
Preparation of Methanol: Methanol can also be prepared as
Hydroxylation of Alkenes
By Fermentation-2/”>Fermentation :
Fermentation is the slow decomposition of complex organic compounds into simpler organic compounds by the activity of ENZYMES. Enzymes are complex, nitrogenous (proteins), non living macro Molecules of high molecular weight derived from living organisms. These are also known as biological catalysts.
Fermentation process is generally accompanied with evolution of gases like CO2 & CH4 and are exothermic in nature.
The alcoholic fermentation involves conversion of sugar into ethyl alcohol by yeast.
The starting material for alcoholic fermentation is starch (potato, rice, barley, maize). The source of starch depends upon its availability in that country. In India, alcoholic fermentation is made by molasses i.e. the dark coloured syrupy liquid left after crystallization of sugar from sugar cane juice. Molasses contains about 50% sugar left after crystallization of sugar from cane juice.
Conditions Favourable for Fermentation
1.Optimum temperature range for fermentation s 25-30oC. At higher temperature enzymes are coagulated.
2.Certain inorganic substances, (NH4)2SO4, phosphate etc are added as food for ferment cells.
3.Solution to be fermented should be dilute.
4.Substances like boric acid, mercury slats etc. should not be present as they retard fermentation.
5.Proper aeration should be maintained in fermentation.
Note : The name fermentation has been derived from Latin word ferver meaning to boil, because during fermentation there is lot of frothing due to evolution of CO2 and this gives the appearance of boiling liquid.
,
The laboratory method of preparing alcohol is a process that involves the fermentation of sugars. The sugars can be derived from a variety of sources, including fruits, vegetables, and grains. The fermentation process is carried out by yeast, which converts the sugars into alcohol and carbon dioxide. The alcohol is then distilled to remove the water and other impurities. The final product is a pure form of alcohol that can be used for a variety of purposes.
The following are the sub topics of the laboratory method of preparing alcohol:
- Fermentation
- Distillation
- Purification
- Storage
- Use
Fermentation is the process by which yeast converts sugars into alcohol and carbon dioxide. The yeast cells produce enzymes that break down the sugars into simpler molecules, which are then converted into alcohol and carbon dioxide. The fermentation process is carried out in a closed container, such as a fermentation vessel or a carboy. The temperature of the fermentation vessel is kept at a constant temperature, typically between 65 and 85 degrees Fahrenheit. The fermentation process can take anywhere from a few days to several weeks, depending on the type of yeast and the sugar concentration.
Distillation is the process of separating a mixture of liquids into its component parts by boiling and then condensing the vapor. The distillation process is carried out in a distillation apparatus, which consists of a condenser, a receiver, and a heating element. The mixture of liquids is heated in the distillation apparatus, and the vapor is then condensed in the condenser. The condensed vapor is collected in the receiver. The distillation process can be used to separate a variety of mixtures, including alcohol and water.
Purification is the process of removing impurities from a substance. The purification process can be carried out by a variety of methods, including distillation, filtration, and crystallization. The purification process is used to produce a pure form of alcohol that can be used for a variety of purposes.
Storage is the process of keeping a substance in a safe and secure location. The storage process can be carried out in a variety of ways, including in a refrigerator, in a freezer, or in a storage container. The storage process is important to ensure that the substance remains in good condition and does not spoil.
Use is the process of putting a substance to a particular purpose. The use of alcohol can vary depending on the type of alcohol and the person consuming it. Alcohol can be used for a variety of purposes, including as a beverage, as a solvent, and as a fuel.
Fermentation
The fermentation process is the first step in the laboratory method of preparing alcohol. The fermentation process is carried out by yeast, which converts sugars into alcohol and carbon dioxide. The yeast cells produce enzymes that break down the sugars into simpler molecules, which are then converted into alcohol and carbon dioxide. The fermentation process is carried out in a closed container, such as a fermentation vessel or a carboy. The temperature of the fermentation vessel is kept at a constant temperature, typically between 65 and 85 degrees Fahrenheit. The fermentation process can take anywhere from a few days to several weeks, depending on the type of yeast and the sugar concentration.
Distillation
The distillation process is the second step in the laboratory method of preparing alcohol. The distillation process is carried out in a distillation apparatus, which consists of a condenser, a receiver, and a heating element. The mixture of liquids is heated in the distillation apparatus, and the vapor is then condensed in the condenser. The condensed vapor is collected in the receiver. The distillation process can be used to separate a variety of mixtures, including alcohol and water.
Purification
The purification process is the third step in the laboratory method of preparing alcohol. The purification process can be carried out by a variety of methods, including distillation, filtration, and crystallization. The purification process is used to produce a pure form of alcohol that can be used for a variety of purposes.
Storage
The storage process is the fourth step in the laboratory method of preparing alcohol. The storage process can be carried out in a variety of ways, including in a refrigerator, in a freezer, or in a storage container. The storage process is important to ensure that the substance remains in good condition and does not spoil.
Use
The use of alcohol can vary depending on the type of alcohol and the person consuming it. Alcohol can be used for a variety of purposes, including as a beverage, as a solvent, and as a fuel.
Alcohol as a beverage
Alcohol is a popular beverage that is consumed by people all over the world. Alcohol is typically consumed in the form of beer, wine, or distilled spirits. Alcohol is a depressant that can have a number of effects on the body, including impaired judgment, coordination, and reflexes. Alcohol can also be addictive.
Alcohol as a solvent
Alcohol is a common solvent that
What is the laboratory method of preparing alcohol?
The laboratory method of preparing alcohol is a process that involves the distillation of fermented plant materials. The first step is to ferment the plant material, which can be done by adding yeast to the material and allowing it to convert the sugars into alcohol. Once the fermentation process is complete, the alcohol is then distilled to separate it from the other components of the fermented material.
What are the advantages of using the laboratory method of preparing alcohol?
The laboratory method of preparing alcohol has several advantages over other methods. First, it is a very efficient method, as it allows for the production of large quantities of alcohol. Second, it is a very safe method, as it does not involve the use of any hazardous chemicals. Third, it is a very versatile method, as it can be used to produce a variety of different types of alcohol.
What are the disadvantages of using the laboratory method of preparing alcohol?
The laboratory method of preparing alcohol also has some disadvantages. First, it is a very expensive method, as it requires the use of specialized equipment. Second, it is a very time-consuming method, as the fermentation and distillation processes can take several days to complete. Third, it is a very complex method, as it requires a high level of technical expertise to perform.
How is the laboratory method of preparing alcohol used in Industry?
The laboratory method of preparing alcohol is used in a variety of industries, including the pharmaceutical industry, the food and beverage industry, and the cosmetics industry. In the pharmaceutical industry, alcohol is used as a solvent and as a preservative. In the food and beverage industry, alcohol is used as an ingredient in alcoholic beverages, as a solvent, and as a preservative. In the cosmetics industry, alcohol is used as a solvent, as a preservative, and as an astringent.
What are the safety precautions that should be taken when using the laboratory method of preparing alcohol?
When using the laboratory method of preparing alcohol, it is important to take the following safety precautions:
- Always wear safety goggles and gloves when handling alcohol.
- Always work in a well-ventilated area.
- Never drink alcohol that has been prepared in a laboratory.
- Always dispose of alcohol properly.
What are the environmental impacts of using the laboratory method of preparing alcohol?
The laboratory method of preparing alcohol can have a number of environmental impacts. The fermentation process can produce greenhouse gases, such as methane and carbon dioxide. The distillation process can produce wastewater, which can contain harmful chemicals. It is important to take steps to minimize these environmental impacts, such as using RENEWABLE ENERGY sources to power the fermentation and distillation processes, and treating wastewater before it is released into the Environment.
Question 1
Which of the following is not a laboratory method of preparing alcohol?
(A) Fermentation
(B) Distillation
(C) Extraction
(D) Sublimation
Answer
(D) Sublimation is not a laboratory method of preparing alcohol. Sublimation is a process by which a solid turns directly into a gas without passing through the liquid state. This process can be used to separate different substances, but it is not used to prepare alcohol.
Question 2
Fermentation is a process by which yeast converts sugar into alcohol and carbon dioxide. Which of the following is a common example of fermentation?
(A) Making wine
(B) Making beer
(C) Making bread
(D) All of the above
Answer (D) All of the above are common examples of fermentation. In winemaking, yeast converts the sugars in grapes into alcohol and carbon dioxide. In beermaking, yeast converts the sugars in barley into alcohol and carbon dioxide. In breadmaking, yeast converts the sugars in flour into alcohol and carbon dioxide, which produces the bubbles that make bread rise.
Question 3
Distillation is a process of separating a mixture of liquids into its component parts by boiling the mixture and then collecting the vapors. Which of the following is a common example of distillation?
(A) Making whiskey
(B) Making vodka
(C) Making gin
(D) All of the above
Answer (D) All of the above are common examples of distillation. In whiskey making, the fermented mash is distilled to produce a high-proof spirit. In vodka making, the fermented mash is distilled to produce a very pure spirit. In gin making, the fermented mash is distilled with juniper berries to produce a flavored spirit.
Question 4
Extraction is a process of separating a substance from a mixture by dissolving it in a solvent. Which of the following is a common example of extraction?
(A) Making essential oils
(B) Making coffee
(C) Making tea
(D) All of the above
Answer (D) All of the above are common examples of extraction. In essential oil making, the plant material is extracted with a solvent to produce a concentrated oil. In coffee making, the coffee beans are extracted with hot water to produce a brewed coffee. In tea making, the tea leaves are extracted with hot water to produce a brewed tea.