In 1897 J.J. Thomson discovered electron as a constituent of atom. He determined that an electron had a negative charge and had very little mass as compared to that of the atom. Since an atom was found to be electrically neutral it was inferred that some source of positive charge must be present in the atom. This soon led to the experimental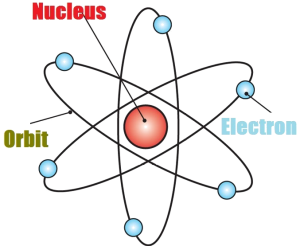
Various theories put forwarded regarding the structure are as follows:-
Dalton’s atomic theory
John Dalton used the Greek concept of an atom and the laws of definite proportions, conservation of mass and multiple proportions to give the atomic theory on scientific basis. Dalton’s atomic theory states that all matter is made of atoms. Atoms are indivisible and indestructible, all atoms of a given element are identical in mass and properties,compounds are formed by a combination of two or more different kinds of atoms and a Chemical Reaction is a rearrangement of atoms.
J . J. Thompson Plum pudding model
The discovery that atoms contained electrons caused Thompson to predict an atomic structure, according to which the whole atom was considered to be a positive sphere,
with negatively charged electrons embedded in it like a plum in a pudding. Thompson’s model did not have any nucleus in it.
But, with the discovery of the nucleus and positively charged proton and neutrally charged neutrons, two more important models of atomic structure were put forward:
Rutherford atomic model
Rutherford atomic model, though a major breakthrough with a central nucleus and surrounding electrons, did not explain how an electron keeps revolving around the nucleus without losing energy.
Bohr’s atomic structure
Niels Bohr expanded Rutherford’s theory further and gave a clear concept of balancing the attractive force and the centrifugal force of the revolving electrons.
The atomic theory put forward by Niel’s Bohr, was completely successful, except for certain corrections, like replacement of the orbits of Bohr by orbitals, etc.,
Atoms are the basic units of matter. They are so small that they cannot be seen with the naked eye, even with the most powerful microscopes. However, atoms are incredibly complex. They are made up of three types of subatomic particles: protons, neutrons, and electrons.
Protons and neutrons are found in the nucleus of the atom, while electrons orbit the nucleus. The number of protons in an atom determines its chemical element. For example, all atoms with one proton in their nucleus are hydrogen atoms, all atoms with two protons are helium atoms, and so on.
The number of neutrons in an atom can vary, even for atoms of the same element. These atoms are called isotopes. For example, most hydrogen atoms have one proton and one neutron, but a small number of hydrogen atoms have one proton and no neutrons. These atoms are called protium, deuturium, and tritium, respectively.
Electrons are negatively charged particles that orbit the nucleus of the atom. The number of electrons in an atom is equal to the number of protons in the nucleus. This is because atoms are electrically neutral, so the positive charge of the protons must be balanced by the negative charge of the electrons.
Electrons are arranged in shells around the nucleus. The first shell can hold up to two electrons, the second shell can hold up to eight electrons, the third shell can hold up to 18 electrons, and so on. The outermost shell is called the valence shell. The electrons in the valence shell are the most important electrons for chemical bonding.
When two atoms bond together, they form a molecule. The atoms in a molecule are held together by chemical Bonds. There are three main types of chemical bonds: covalent bonds, ionic bonds, and metallic bonds.
Covalent bonds are formed when atoms share electrons. In a covalent bond, the atoms overlap their valence shells and share the electrons in the overlapping region. This type of bond is very strong and is found in many different types of Molecules, including water, methane, and carbon dioxide.
Ionic bonds are formed when atoms transfer electrons to each other. In an ionic bond, one atom becomes positively charged and the other atom becomes negatively charged. The positive and negative ions are then attracted to each other, forming a bond. This type of bond is found in salt, table sugar, and many other compounds.
Metallic bonds are formed when atoms share electrons in a sea of electrons. In a metallic bond, the atoms in the Metal are held together by a Network of delocalized electrons. These electrons are free to move throughout the metal, which is why metals are good Conductors of electricity.
Orbitals are regions of space around the nucleus where electrons are likely to be found. Orbitals are described by four quantum numbers: the principal quantum number (n), the azimuthal quantum number (l), the magnetic quantum number (m), and the spin quantum number (s).
The principal quantum number (n) determines the size of the orbital. The higher the value of n, the larger the orbital. The azimuthal quantum number (l) determines the shape of the orbital. The values of l can range from 0 to n-1. The magnetic quantum number (m) determines the orientation of the orbital in space. The values of m can range from -l to l, including 0. The spin quantum number (s) can have the values of +1/2 or -1/2.
The Aufbau principle states that electrons fill orbitals in order of increasing energy. The Hund’s rule states that electrons will occupy degenerate orbitals (orbitals with the same energy) singly before pairing up. The Pauli exclusion principle states that no two electrons in an atom can have the same set of four quantum numbers.
The periodic table is a table of the Elements that is arranged in order of increasing atomic number. The elements in the periodic table are grouped into groups and periods. The groups are columns in the periodic table, and the periods are rows in the periodic table.
The representative elements are the elements in groups 1, 2, and 13-18 of the periodic table. These elements are all metals except for hydrogen and helium. The transition metals are the elements in groups 3-12 of the periodic table. These elements are all metals. The inner transition metals are the elements in the f-block of the periodic table. These elements are all metals. The metalloids are the elements that border the metals and nonmetals in the periodic table. These elements have properties of both metals and nonmetals. The nonmetals are the elements in groups 15-18 of the periodic table. These elements are all nonmetals except for boron and silicon. The noble gases are the elements in group 18 of the periodic table. These elements are all nonmetals and are very stable.
The chemical properties of an element are
What is an atom?
An atom is the smallest unit of ordinary matter that forms a chemical element. Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Atoms are extremely small, typically around 100 picometers across. They are so small that accurately predicting their behavior using classical physics is impossible. Instead, quantum mechanics must be used to correctly describe and predict their behavior.
What are the parts of an atom?
The three main parts of an atom are the nucleus, the electron cloud, and the electron shells. The nucleus is made up of protons and neutrons, which are held together by the strong force. The electron cloud is a region around the nucleus where electrons are found. The electron shells are regions around the nucleus where electrons are most likely to be found.
What are the properties of atoms?
The properties of atoms are determined by the number of protons, neutrons, and electrons they have. The number of protons in the nucleus determines the element of the atom. For example, all atoms with one proton in the nucleus are hydrogen atoms. The number of neutrons in the nucleus determines the isotope of the atom. For example, there are three isotopes of hydrogen: protium, deuterium, and tritium. The number of electrons in the atom determines its chemical properties.
How do atoms interact with each other?
Atoms interact with each other through chemical bonds. Chemical bonds are forces that hold atoms together. There are four types of chemical bonds: ionic bonds, covalent bonds, metallic bonds, and van der Waals forces. Ionic bonds are formed when atoms transfer electrons to each other. Covalent bonds are formed when atoms share electrons. Metallic bonds are formed when atoms share electrons in a sea of electrons. Van der Waals forces are weak forces that are formed when atoms are close to each other.
What are the different types of atoms?
There are over 118 elements in the periodic table, each with its own unique set of properties. The elements are arranged in the periodic table by their atomic number, which is the number of protons in the nucleus. The elements are also grouped into families based on their chemical properties. For example, the alkali metals are all very reactive metals that are found in Group 1 of the periodic table.
What is the history of atomic theory?
The history of atomic theory is a long and winding one. The first person to propose that matter was made up of tiny particles was Democritus in the 5th century BC. However, it wasn’t until the 17th century that scientists began to seriously study the nature of atoms. In 1661, Robert Hooke proposed that atoms were tiny, solid spheres. In 1789, Antoine Lavoisier proposed that all matter was made up of atoms of different elements. In 1803, John Dalton proposed that atoms were indivisible and indestructible. In 1897, J.J. Thomson discovered the electron, which showed that atoms were not indivisible after all. In 1911, Ernest Rutherford discovered the nucleus, which showed that atoms were made up of a central nucleus surrounded by electrons. In 1913, Niels Bohr proposed the Bohr model of the atom, which showed that electrons orbit the nucleus in specific energy levels. In 1926, Erwin Schrödinger developed the Schrödinger equation, which showed that electrons can be found in a Probability cloud around the nucleus. In 1932, James Chadwick discovered the neutron, which showed that the nucleus was made up of protons and neutrons. In 1961, Murray Gell-Mann proposed the quark model, which showed that protons and neutrons are made up of quarks.
What is the future of atomic theory?
The future of atomic theory is bright. Scientists are still Learning about the nature of atoms and how they interact with each other. This knowledge is being used to develop new technologies, such as nuclear power and quantum computing.
The smallest unit of matter that retains all of the chemical properties of an element is called an:
(A) atom
(B) molecule
(C) ion
(D) compoundThe nucleus of an atom is made up of:
(A) protons and neutrons
(B) electrons and neutrons
(C) protons and electrons
(D) neutrons and electronsThe number of protons in the nucleus of an atom is called the:
(A) atomic number
(B) mass number
(C) charge number
(D) atomic massThe number of neutrons in the nucleus of an atom is called the:
(A) atomic number
(B) mass number
(C) charge number
(D) atomic massThe number of protons and neutrons in the nucleus of an atom is called the:
(A) atomic number
(B) mass number
(C) charge number
(D) atomic massAtoms of the same element with different numbers of neutrons are called:
(A) isotopes
(B) ions
(C) molecules
(D) compoundsAtoms of different elements that have the same number of protons are called:
(A) isotopes
(B) ions
(C) molecules
(D) compoundsAtoms of different elements that have the same number of electrons are called:
(A) isotopes
(B) ions
(C) molecules
(D) compoundsAtoms of different elements that have the same number of neutrons are called:
(A) isotopes
(B) ions
(C) molecules
(D) compoundsAtoms that have lost or gained electrons are called:
(A) isotopes
(B) ions
(C) molecules
(D) compounds