Alloy
You might see the word alloy described as a “mixture of metals”, but that’s a little bit misleading because some alloys contain only one Metal and it’s mixed in with other substances that are nonmetals (cast iron, for example, is an alloy made of just one metal, iron, mixed with one nonmetal, carbon). The best way to think of an alloy is as a material that’s made up of at least two different chemical Elements, one of which is a metal. The other components of an alloy (which are called alloying agents) can be either metals or nonmetals and they’re present in much smaller quantities (sometimes less than 1 percent of the total). Although an alloy can sometimes be a compound (the elements it’s made from are chemically bonded together), it’s usually a solid solution (atoms of the elements are simply intermixed, like salt mixed with water).component of an alloy (often representing 90 percent or more of the material) is called the
The structure of alloys
If you look at a metal through a powerful electron Microscope, you can see the atoms inside arranged in a regular structure called a crystalline lattice. Imagine a small cardboard box full of marbles and that’s pretty much what you’d see. In an alloy, apart from the atoms of the main metal, there are also atoms of the alloying agents dotted throughout the structure. 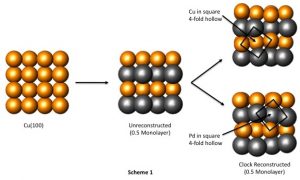
Substitution alloys
If the atoms of the alloying agent replace atoms of the main metal, we get what’s called a substitution alloy. An alloy like this will form only if the atoms of the base metal and those of the alloying agent are of roughly similar size. In most substitution alloys, the constituent elements are quite near one another in the periodic table. Brass, for example, is a substitution alloy based on copper in which atoms of zinc replace 10–35 percent of the atoms that would normally be in copper. Brass works as an alloy because copper and zinc are close to one another in the periodic table and have atoms of roughly similar size.
Interstitial alloys
Alloys can also form if the alloying agent or agents have atoms that are very much smaller than those of the main metal. In that case, the agent atoms slip in between the main metal atoms (in the gaps or “interstices”), giving what’s called an interstitial alloy. Steel is an example of an interstitial alloy in which a relatively small number of carbon atoms slip in the gaps between the huge atoms in a crystalline lattice of iron.
How do alloys behave?
People make and use alloys because metals don’t have exactly the right properties for a particular job. Iron is a great building material but steel (an alloy made by adding small amounts of nonmetallic carbon to iron) is stronger, harder, and rustproof. Aluminum is a very Light metal but it’s also very soft in its pure form. Add small amounts of the metals magnesium, manganese, and copper and you make a superb aluminum alloy called duralumin, which is strong enough to make airplanes. Alloys always show improvements over the main metal in one or more of their important physical properties (things like strength, durability, ability to conduct electricity, ability to withstand heat, and so on). Generally, alloys are stronger and harder than their main metals, less malleable (harder to work) and less ductile (harder to pull into wires).
How are alloys made?
You might find the idea of an alloy as a “mixture of metals” quite confusing. How can you mix together two lumps of solid metal? The traditional way of making alloys was to heat and melt the components to make liquids, mix them together, and then allow them to cool into what’s called a solid solution (the solid equivalent of a solution like salt in water). An alternative way of making an alloy is to turn the components into powders, mix them together, and then fuse them with a combination of high pressure and high temperature. This technique is called powder metallurgy. A third method of making alloys is to fire beams of ions (atoms with too few or too many electrons) into the surface layer of a piece of metal. Ion implantation, as this is known, is a very precise way of making an alloy. It’s probably best known as a way of making the semiconductors used in electronic circuits and computer chips. (Read more about this in our ARTICLE on molecular beam epitaxy.)
Some Common Alloys
There are zillions of different alloys used for zillions of different purposes. We’ve listed 20 of the more common (or otherwise interesting) ones in the table below. There are lots of different variations on most alloys and the precise mixture can vary widely, so the Percentage figures you see quoted in different books will often not agree exactly.
| Alloy | Components | Typical uses |
| Alnico | Iron (50%+), aluminum (8–12%), nickel (15–25%), cobalt (5–40%), plus other metals such as copper and titanium. | Magnets in loudspeakers and pickups in electric guitars. |
| Amalgam | Mercury (45–55%), plus silver, tin, copper, and zinc. | Dental fillings. |
| Babbitt metal (“white metal”) | Tin (90%), antimony (7–15%), copper (4–10%). | Friction-reducing coating in machine bearings. |
| Brass | Copper (65–90%), zinc (10–35%). | Door locks and bolts, brass Musical instruments, central heating pipes. |
| Bronze | Copper (78–95%), tin (5–22%), plus manganese, phosphorus, aluminum, or silicon. | Decorative statues, musical instruments. |
| Cast iron | Iron (96–98%), carbon (2–4%), plus silicon. | Metal structures such as bridgesand heavy-duty cookware. |
| Cupro-nickel (copper nickel) | Copper (75%), nickel (25%), plus small amounts of manganese. | Coins. |
| Duralumin | Aluminum (94%), copper (4.5–5%), magnesium (0.5–1.5%), manganese (0.5–1.5%). | Automobile and aircraft body parts, military equipment. |
| German Silver | Copper+Nickel+Zinc | Tableware,Marine Fittings and Plumbering |
| Gunmetal | Copper (80–90%), tin (3–10%), zinc (2–3%), and phosphorus. | Guns, decorative items. |
| Magnox | Magnesium, aluminum. | Nuclear reactors. |
| Nichrome | Nickel (80%), chromium (20%). | Firework ignition devices, heating elements in electrical appliances. |
| Nitinol | Nickel (50–55%), titanium (45–50%). | Shape-memory alloy used in medical items, spectacle frames that spring back to shape, and temperature switches. |
| Pewter | Tin (80–99%) with copper, lead, and antimony. | Ornaments, used to make tableware before glass became more common. |
| Solder | Varies. Old-fashioned solders contain a mixture of tin (50-70%), lead (30-50%), copper, antimony, and other metals. Newer solders dispense with lead for Health reasons. A typical modern solder has 99.25 percent tin and 0.75 percent copper. | Connecting electrical components into circuits. |
| Steel (general) | Iron (80–98%), carbon (0.2–2%), plus other metals such as chromium, manganese, and vanadium. | Metal structures, car and airplane parts, and many other uses. |
| Steel (stainless) | Iron (50%+), chromium (10–30%), plus smaller amounts of carbon, nickel, manganese, molybdenum, and other metals. | Jewelry, medical tools, tableware. |
| Stellite | Cobalt (67%), chromium (28%), tungsten (4%), nickel (1%). | Coating for cutting tools such as saw teeth, lathes, and chainsaws. |
| Sterling silver | Silver (92.5%), copper (7.5%). | Cutlery, jewelry, medical tools, musical instruments. |
| White gold (18 carat) | Gold (75%), palladium (17%), silver (4%), copper (4%) | Jewelry. |
| Wood’s metal | Bismuth (50%), lead (26.7%), tin (13.3%), cadmium (10%). | Solder, melting element in fire sprinkler systems. |
,
An alloy is a mixture of two or more metals, or of a metal and a nonmetal, that has metallic properties. Alloys are typically harder and stronger than the pure metals from which they are made. They are also often more resistant to corrosion and wear.
Alloys are made by melting the metals together and then cooling them slowly. The cooling process allows the atoms of the different metals to form a solid solution, which is a mixture of atoms that are evenly distributed throughout the alloy.
The properties of an alloy depend on the types of metals that are used and on the proportions in which they are mixed. For example, adding small amounts of carbon to iron makes it much harder and stronger. This is why steel, which is an alloy of iron and carbon, is used to make tools and machines.
Alloys are used in a wide variety of applications, including construction, manufacturing, and transportation. They are also used in jewelry, coins, and other decorative items.
Some common alloys include:
- Aluminum alloys are lightweight and strong, and they are used in a variety of applications, including aircraft, cars, and buildings.
- Beryllium alloys are very strong and lightweight, and they are used in applications where weight is a critical factor, such as aerospace and nuclear power.
- Bronze is an alloy of copper and tin, and it is used in a variety of applications, including sculptures, musical instruments, and coins.
- Cast iron is an alloy of iron and carbon, and it is used in a variety of applications, including cookware, engine blocks, and railroad tracks.
- Cobalt alloys are strong and corrosion-resistant, and they are used in a variety of applications, including magnets, jet engines, and medical implants.
- Copper alloys are ductile and easy to work with, and they are used in a variety of applications, including plumbing, electrical wiring, and jewelry.
- Gold alloys are soft and malleable, and they are used in a variety of applications, including jewelry, coins, and dental fillings.
- Iron alloys are strong and durable, and they are used in a variety of applications, including buildings, bridges, and cars.
- Lead alloys are soft and easy to cast, and they are used in a variety of applications, including batteries, ammunition, and solder.
- Magnesium alloys are lightweight and strong, and they are used in a variety of applications, including aircraft, cars, and Sports equipment.
- Manganese alloys are strong and tough, and they are used in a variety of applications, including steelmaking, mining, and construction.
- Nickel alloys are strong, corrosion-resistant, and magnetic, and they are used in a variety of applications, including coins, jewelry, and electrical equipment.
- Niobium alloys are strong and ductile, and they are used in a variety of applications, including superconductors, jet engines, and medical implants.
- Palladium alloys are white and lustrous, and they are used in a variety of applications, including jewelry, dentistry, and electronics.
- Platinum alloys are white and lustrous, and they are used in a variety of applications, including jewelry, dentistry, and electronics.
- Potassium alloys are soft and easily melted, and they are used in a variety of applications, including fireworks, flares, and batteries.
- Silver alloys are white and lustrous, and they are used in a variety of applications, including jewelry, silverware, and electronics.
- Sodium alloys are soft and easily melted, and they are used in a variety of applications, including fireworks, flares, and batteries.
- Steel is an alloy of iron and carbon, and it is one of the most widely used materials in the world. It is used in a variety of applications, including construction, manufacturing, and transportation.
- Titanium alloys are strong, lightweight, and corrosion-resistant, and they are used in a variety of applications, including aircraft, spacecraft, and medical implants.
- Tungsten alloys are strong, hard, and wear-resistant, and they are used in a variety of applications, including cutting tools, electrical contacts, and X-ray tubes.
- Zinc alloys are strong, corrosion-resistant, and easy to cast, and they are used in a variety of applications, including batteries, plumbing, and hardware.
What is a metal?
A metal is a chemical element that is typically hard, shiny, and has a high melting point. Metals are good Conductors of heat and electricity. They are also ductile, meaning they can be easily shaped.
What are the different types of metals?
There are many different types of metals, including iron, copper, aluminum, and gold. Each type of metal has its own unique properties. For example, iron is a strong metal that is often used in construction. Copper is a good conductor of electricity and is often used in electrical wiring. Aluminum is a lightweight metal that is often used in aircraft. Gold is a precious metal that is often used in jewelry.
What are the properties of metals?
Metals are typically hard, shiny, and have a high melting point. They are also good conductors of heat and electricity. Metals are ductile, meaning they can be easily shaped.
What are some common uses for metals?
Metals are used in a wide variety of products, including cars, buildings, appliances, and jewelry. They are also used in many industrial processes.
What are some of the benefits of using metals?
Metals are strong, durable, and versatile. They are also good conductors of heat and electricity.
What are some of the drawbacks of using metals?
Metals can be expensive. They can also be heavy and difficult to work with.
What are some of the environmental impacts of using metals?
Mining and processing metals can have a negative impact on the Environment. Metals can also be a source of pollution.
What are some of the safety concerns associated with using metals?
Metals can be sharp and can cause cuts. They can also be hot and can cause burns.
What are some of the recycling Options for metals?
Metals can be recycled many times. Recycling metals helps to conserve Resources and reduce pollution.
What is the future of metals?
The future of metals is bright. Metals are essential for many products and Services. As the world’s Population grows, the demand for metals will continue to increase.
Question 1
Which of the following is not a type of metal?
(A) Iron
(B) Copper
(C) Gold
(D) Alloy
Answer
(D) Alloy is a mixture of two or more metals. The other options are all pure metals.
Question 2
Which of the following is the most common metal in the Earth’s crust?
(A) Iron
(B) Aluminum
(C) Silicon
(D) Oxygen
Answer
(A) Iron is the most common metal in the Earth’s crust, making up about 5% of the total mass. Aluminum is the second most common metal, making up about 8% of the total mass. Silicon is the third most common element in the Earth’s crust, making up about 28% of the total mass. Oxygen is the most common element in the Earth’s crust, making up about 46% of the total mass.
Question 3
Which of the following is the most ductile metal?
(A) Gold
(B) Silver
(C) Copper
(D) Platinum
Answer
(A) Gold is the most ductile metal. It can be stretched into very thin wires or sheets. Silver is the second most ductile metal, followed by copper and platinum.
Question 4
Which of the following is the most malleable metal?
(A) Gold
(B) Silver
(C) Copper
(D) Platinum
Answer
(A) Gold is the most malleable metal. It can be hammered into very thin sheets. Silver is the second most malleable metal, followed by copper and platinum.
Question 5
Which of the following is the most conductive metal?
(A) Silver
(B) Copper
(C) Gold
(D) Aluminum
Answer
(A) Silver is the most conductive metal. It is followed by copper, gold, and aluminum.
Question 6
Which of the following is the most thermally conductive metal?
(A) Silver
(B) Copper
(C) Gold
(D) Aluminum
Answer
(A) Silver is the most thermally conductive metal. It is followed by copper, gold, and aluminum.
Question 7
Which of the following is the most corrosion-resistant metal?
(A) Gold
(B) Silver
(C) Copper
(D) Platinum
Answer
(A) Gold is the most corrosion-resistant metal. It is followed by silver, copper, and platinum.
Question 8
Which of the following is the strongest metal?
(A) Tungsten
(B) Titanium
(C) Iron
(D) Steel
Answer
(A) Tungsten is the strongest metal. It is followed by titanium, iron, and steel.
Question 9
Which of the following is the lightest metal?
(A) Lithium
(B) Beryllium
(C) Magnesium
(D) Aluminum
Answer
(A) Lithium is the lightest metal. It is followed by beryllium, magnesium, and aluminum.
Question 10
Which of the following is the heaviest metal?
(A) Gold
(B) Platinum
(C) Iridium
(D) Osmium
Answer
(D) Osmium is the heaviest metal. It is followed by iridium, platinum, and gold.