In chemistry, a metal is an element that readily forms positive ions (cations) and has metallic Bonds. Metals are sometimes described as a lattice of positive ions surrounded by a cloud of delocalized electrons. The metals are one of the three groups of Elements as distinguished by their ionization and bonding properties, along with the metalloids and nonmetals. On the periodic table, a diagonal line drawn from boron (B) to polonium (Po) separates the metals from the nonmetals. Most elements on this line are metalloids, sometimes called semi-metals; elements to the lower left are metals; elements to the upper right are nonmetals. A modern definition of metals is that they have overlapping conduction bands and valence bands in their electronic structure. This definition opens up the category for metallic polymers and other organic metals, which have been made by researchers and employed in high-tech devices. These synthetic materials often have the characteristic silvery-grey reflectiveness of elemental metals. The traditional definition focuses on the bulk properties of metals. They tend to be lustrous, ductile, malleable, and good Conductors of electricity, while nonmetals are generally brittle (for solid nonmetals), lack lustre, and are insulators.
In the periodic table, you can see a stair-stepped line starting at Boron (B), atomic number 5, and going all the way down to Polonium (Po), atomic number 84. Except for Germanium (Ge) and Antimony (Sb), all the elements to the left of that line can be classified as metals. These metals have properties that you normally associate with the metals you encounter in everyday life:
- They are solid (with the exception of mercury, Hg, a liquid).
- They are shiny, good conductors of electricity and heat.
- They are ductile(they can be drawn into thin wires).
- They are malleable(they can be easily hammered into very thin sheets).
All these metals tend to lose electrons easily. The following figure shows the metals.
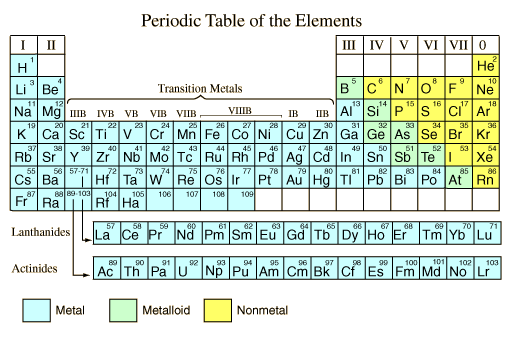
As far as elements are concerned, a nonmetal is simply an element that does not display the properties of a metal. It is not defined by what it is, but by what it is not. It doesn’t look metallic, can’t be drawn into a wire or pounded into shape or bent, doesn’t conduct heat or electricity well, and doesn’t have a high melting or boiling point.
The nonmetals are in the minority on the periodic table, mostly pushed to the right hand side of the periodic table.
The exception is hydrogen, which behaves as a nonmetal at room temperature and pressure and is found on the upper left corner of the periodic table. Under conditions of high pressure, hydrogen is predicted to
behave as an alkali metal.Here’s a look at which elements are nonmetals, how to locate the nonmetals on the table, and their common properties.
LOCATION ON THE NONMETALS ON THE PERIODIC TABLE
The nonmetals are located on the upper right side of the periodic table. Nonmetals are separated from metals by a line that cuts diagonally through the region of the periodic table containing elements with partially filled p orbitals. The halogens and noble gasesare nonmetals, but the nonmetal element group usually is considered to consist of the following elements:
- hydrogen
- carbon
- nitrogen
- Oxygen
- phosphorus
- sulfur
- selenium
The halogen elements are:
- fluorine
- chlorine
- bromine
- iodine
- astatine
- Possibly element 117 (tennessine), although most scientists think this element will behave as a metalloid.
The noble gas elements are:
- helium
- neon
- argon
- krypton
- xenon
- radon
- element 118 – oganesson (predicted to be a liquid, but still a nonmetal)
PROPERTIES OF NONMETALS
Nonmetals have high ionization energies and electronegativities. They are generally poor conductors of heat and electricity. Solid nonmetals are generally brittle, with little or no metallic luster.
Most nonmetals have the ability to gain electrons easily. Nonmetals display a wide range of chemical properties and reactivities.
SUMMARY OF COMMON PROPERTIES
- High ionization energies
- High electronegativities
- Poor thermal conductors
- Poor electrical conductors
- Brittle solids – not malleable or ductile
- Little or no metallic luster
- Gain electrons easily
- Dull, not metallic-shiny, although they may be colorful
- Lower melting points and boiling point than the metals
COMPARING THE METALS AND NONMETALS
Here’s a comparison of the physical and chemical properties of the metals and nonmetals. These properties apply to the metals in general (alkali metals, alkaline earth, transition metals, basic metals, lanthanides, actinides) and nonmetals in general (nonmetals, halogens, noble gases).
| Metals | Nonmetals | |
| chemical properties | easily lose valence electrons | easily share or gain valence electrons |
| 1-3 electrons (usually) in the outer shell | 4-8 electrons in the outer shell (7 for halogens and 8 for noble gases) | |
| form basic oxides | form acidic oxides | |
| good reducing agents | good oxidizing agents | |
| have low electronegativity | have higher electronegativity | |
| physical properties | solid at room temperature (except mercury) | may be liquid, solid, or gas (noble gases are gases) |
| have metallic luster | do not have metallic luster | |
| good conductor of heat and electricity | poor conductor of heat and electricity | |
| typically malleable and ductile | usually brittle | |
| opaque in a thin sheet | transparent in a thin sheet |
,
Metals and non-metals are two of the main types of elements in the periodic table. Metals are typically shiny, ductile, and good conductors of heat and electricity. Non-metals are typically dull, brittle, and poor conductors of heat and electricity.
- Alloys are mixtures of two or more metals. They are often used to improve the properties of the individual metals. For example, adding small amounts of carbon to iron makes it stronger and harder, resulting in steel.
- Amorphous metals are metals that do not have a regular crystal structure. They are often used in applications where they need to be flexible, such as in electronics.
- Brittle metals are metals that are easily broken. They are often used in applications where they need to be strong, but not flexible, such as in construction.
- Conductivity is the ability of a material to carry an electric current. Metals are good conductors of electricity because they have free electrons that can move easily.
- Ductility is the ability of a material to be drawn out into a wire. Metals are ductile because they are made up of atoms that are bonded together in a way that allows them to slide past each other.
- Electrical conductivity is the ability of a material to conduct an electric current. Metals are good conductors of electricity because they have free electrons that can move easily.
- Malleability is the ability of a material to be hammered or rolled into thin sheets. Metals are malleable because they are made up of atoms that are bonded together in a way that allows them to slide past each other.
- Metallic bonding is the type of bonding that occurs between atoms in metals. It is caused by the attraction between the positively charged nuclei of the atoms and the negatively charged electrons that are shared between them.
- Metallic luster is the characteristic shine of metals. It is caused by the way that Light interacts with the free electrons in the metal.
- Non-metals are elements that do not have the properties of metals. They are typically dull, brittle, and poor conductors of heat and electricity.
- Physical properties of metals include their luster, malleability, ductility, and conductivity.
- Properties of metals include their luster, malleability, ductility, conductivity, and reactivity.
- Reactivity is the ability of an element to combine with other elements. Metals are typically more reactive than non-metals.
- Thermal conductivity is the ability of a material to conduct heat. Metals are good conductors of heat because they have free electrons that can move easily.
- Valence electrons are the electrons in an atom that are located in the outermost shell. They are responsible for the chemical properties of the atom.
- Workability is the ability of a material to be shaped without breaking. Metals are workable because they are ductile and malleable.
Metals and non-metals are used in a wide variety of applications. Metals are used in construction, transportation, electronics, and many other industries. Non-metals are used in batteries, plastics, and other materials.
Metals and non-metals are essential to our modern world. They are used in everything from buildings to cars to computers. Without metals and non-metals, our lives would be very different.
1. What are the different Types of Rocks?
There are three main types of rocks: igneous, sedimentary, and metamorphic. Igneous Rocks are formed when magma or lava cools and solidifies. Sedimentary Rocks are formed when pieces of other rocks are broken down and deposited over time. Metamorphic Rocks are formed when existing rocks are subjected to heat and pressure.
2. What are the different types of Minerals?
Minerals are naturally occurring, inorganic solids with a definite chemical composition and crystal structure. There are over 4,000 known minerals, but only a few are common. The most common minerals are quartz, feldspar, and mica.
3. What are the different types of fossils?
Fossils are the remains or traces of ancient organisms that have been preserved in rocks. Fossils can be of Plants, animals, or even bacteria. They can be found in all parts of the world, and they provide valuable information about the history of life on Earth.
4. What are the different types of Ecosystems?
An ecosystem is a community of living organisms and their physical Environment. Ecosystems can be very small, such as a pond, or very large, such as a forest. They can also be aquatic, such as a coral reef, or terrestrial, such as a desert.
5. What are the different types of energy?
Energy is the ability to do work. There are many different types of energy, including kinetic energy, potential energy, electrical energy, and chemical energy. Energy can be converted from one form to another, but it cannot be created or destroyed.
6. What are the different types of waves?
Waves are disturbances that travel through a medium, such as water, air, or even solid objects. There are many different types of waves, including water waves, Sound waves, and light waves. Waves can be classified by their wavelength, frequency, and amplitude.
7. What are the different types of forces?
Forces are interactions that cause objects to change their motion. There are many different types of forces, including gravity, friction, and air resistance. Forces can be classified as either contact forces or field forces.
8. What are the different types of motion?
Motion is the change in position of an object over time. There are many different types of motion, including linear motion, circular motion, and oscillatory motion. Motion can be described by its speed, velocity, and acceleration.
9. What are the different types of shapes?
Shapes are the outlines of two-dimensional or three-dimensional objects. There are many different types of shapes, including circles, squares, triangles, and spheres. Shapes can be classified by their symmetry, their dimensions, and their properties.
10. What are the different types of colors?
Colors are the different sensations that we see when light of different wavelengths hits our eyes. There are many different types of colors, including red, orange, yellow, green, blue, indigo, and violet. Colors can be classified by their hue, saturation, and brightness.
Sure, here are some MCQs without mentioning the topic Metals and Non-metals:
-
Which of the following is a good conductor of heat and electricity?
(A) Wood
(B) Plastic
(C) Copper
(D) Water -
Which of the following is a good insulator of heat and electricity?
(A) Wood
(B) Plastic
(C) Copper
(D) Water -
Which of the following is a solid at room temperature?
(A) Mercury
(B) Bromine
(C) Carbon dioxide
(D) Sodium -
Which of the following is a liquid at room temperature?
(A) Mercury
(B) Bromine
(C) Carbon dioxide
(D) Sodium -
Which of the following is a gas at room temperature?
(A) Mercury
(B) Bromine
(C) Carbon dioxide
(D) Sodium -
Which of the following is a metalloid?
(A) Silicon
(B) Germanium
(C) Arsenic
(D) All of the above -
Which of the following is a metal?
(A) Iron
(B) Copper
(C) Gold
(D) All of the above -
Which of the following is a non-metal?
(A) Carbon
(B) Oxygen
(C) Nitrogen
(D) All of the above -
Which of the following is a compound?
(A) Water
(B) Sugar
(C) Salt
(D) All of the above -
Which of the following is an element?
(A) Hydrogen
(B) Oxygen
(C) Nitrogen
(D) All of the above
I hope these MCQs are helpful!